Search
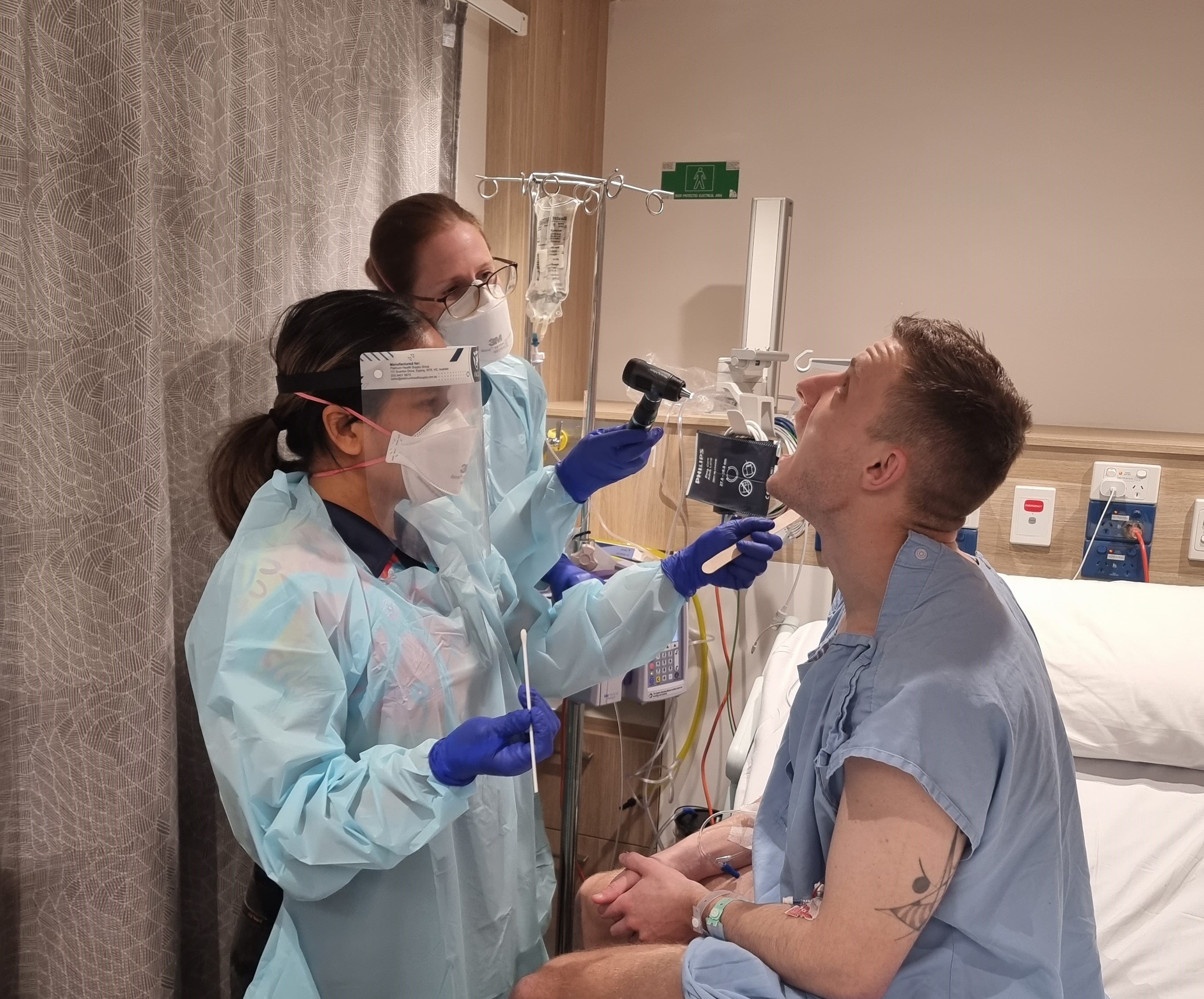
A unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).

A new study underway at the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, is deliberately infecting tonsils with Strep A in the laboratory to test a range of potential vaccine candidates.

National research led by the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, has secured more than $3.4 million to assess the epidemiology of respiratory syncytial virus (RSV) throughout the country and optimise Australia’s immunisation strategy.
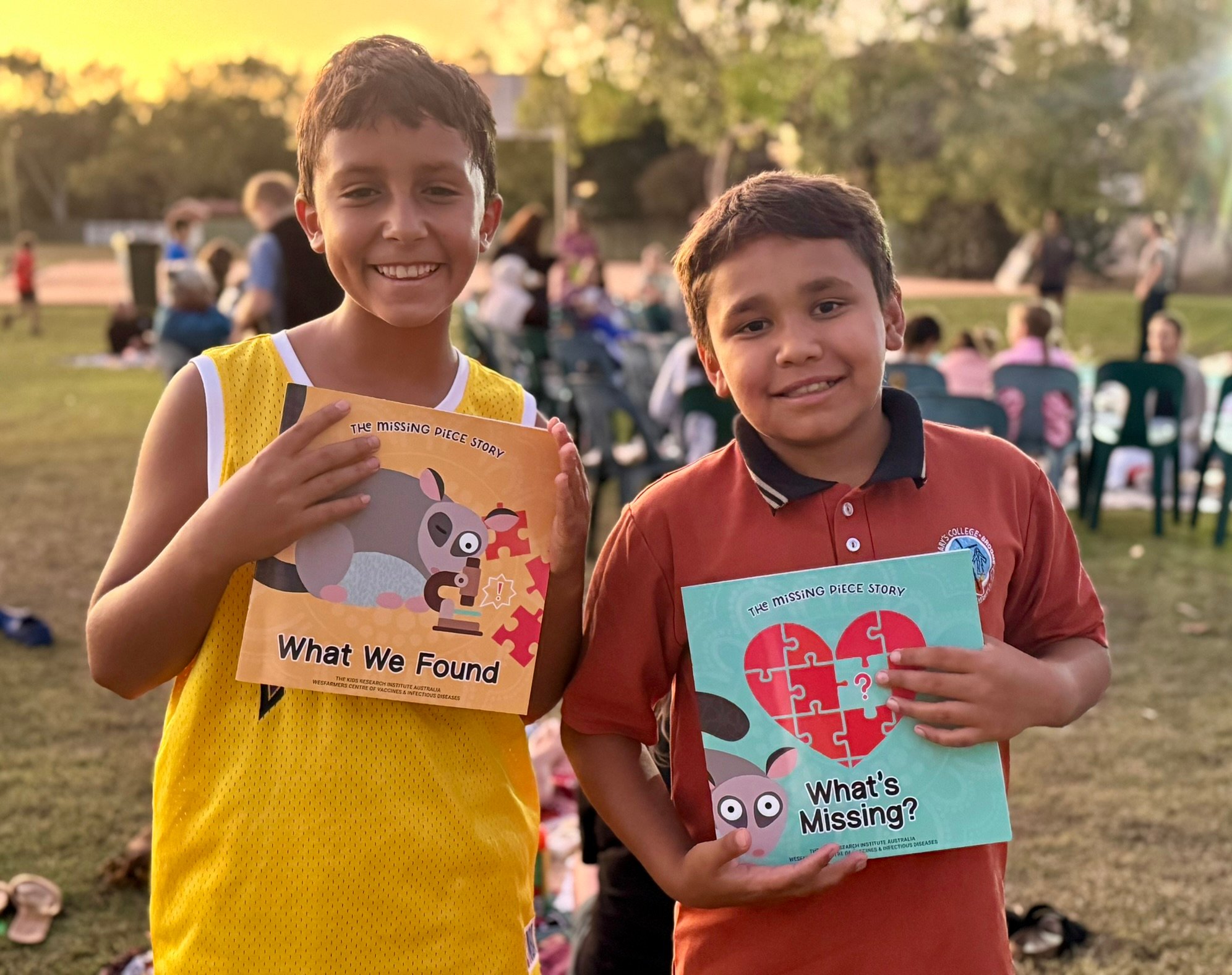
Wesfarmers Centre of Vaccines and Infectious Diseases researchers Dr Janessa Pickering and Dr August Mikucki travelled to Broome last week for the official launch of the long-awaited Missing Piece story books.

Perth investigators involved in a major global trial have launched an innovative Cultural Information Hub to maximise cultural safety for Aboriginal and/or Torres Strait Islander patients participating in research.

10,000 families participating in research by The Kids Research Institute Australia have demonstrated the effectiveness of a simple text message for increasing the number of children receiving their vaccinations on time.

Many parents may be feeling anxious and confused about what COVID-19 means for pregnant women, babies and children.
Learn more about the Wesfarmers Centre of Vaccines and Infectious Diseases
The Vaccine Trials Group was formed in March 1999 to provide a coordinated approach to the development, delivery, assessment and promotion of vaccines.
To improve the health of the community through immunisation and prevention of infectious diseases, we are conducting clinical trials of vaccines.
