Search

News & Events
Camp volunteers neededDiabetes WA is looking for volunteers to take part in the PMH and Diabetes WA Camp for 9 and 10 year olds in Hillarys in September.

News & Events
Pump newsPump update: New changes to health insurance premiums, pump information evening and more

News & Events
Does your school pass the test?Do you feel your child is well supported by their school when it comes to managing diabetes? If so, our researchers want to hear from you.

Mental health and wellness is critical to the overall wellbeing of a person, and can also impact on physical health. Researchers are exploring the mental wellbeing of mothers and their experience of motherhood and pregnancy, and effective support options available. Mental health development of the child is also explored, as well as the impact of the COVID-19 pandemic on our ORIGINS families.
Get in Touch Dropping off a sample or attending a Kids Check appointment? Visit us at our Edgewater clinic. The Kids Joondalup Shop 51, Joondalup

The Happy Parenting Program is investigating new ways to provide support to parents with young children from an early age.
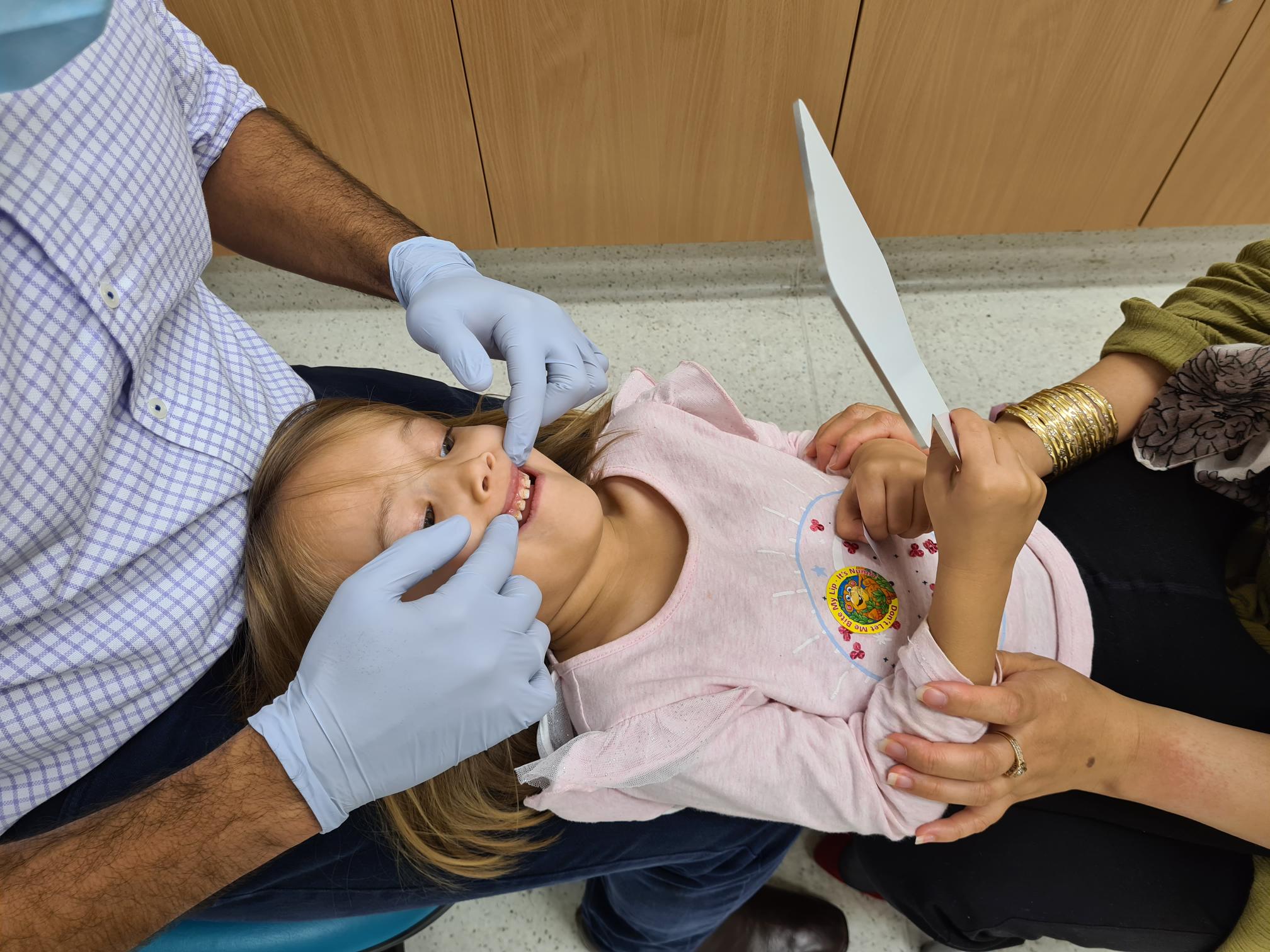
Assessing a dental photographic method as an alternative dental screening method.
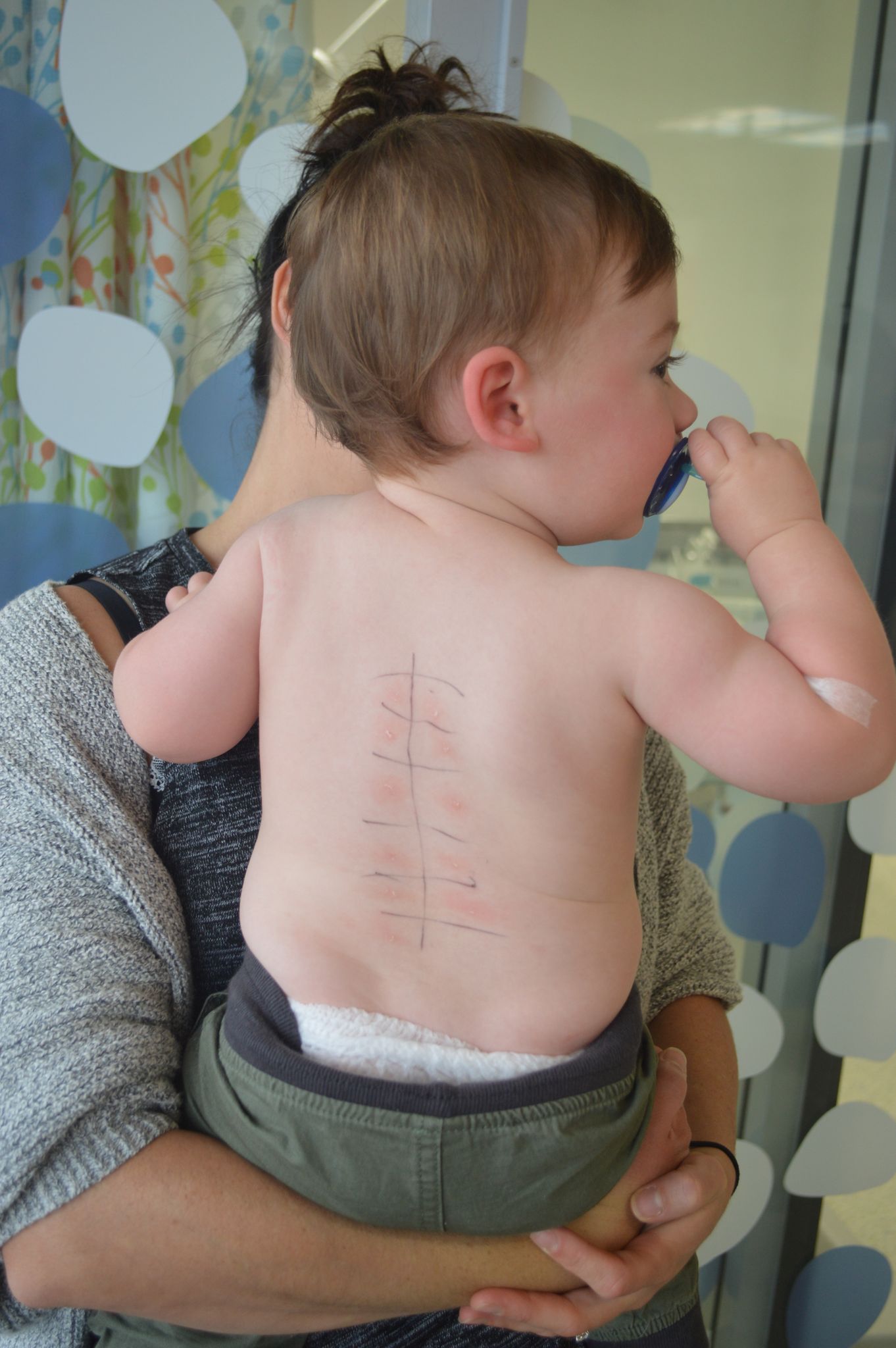
Comparing how mast cells are “programmed” in allergic and non-allergic children at one year of age.

News & Events
ORIGINS takes part in Aboriginal wellness expoORIGINS participated in the inaugural Wadjak Northside Social Emotional Wellbeing Expo on 24 October at the Wadjak Northside Aboriginal Community Centre.

News & Events
Digital support engagement needed for new MumsSystematic review explores engagement levels of digital tools during the perinatal period
